Abstract
Introduction: MCL is an aggressive lymphoma accounting for 6% of all newly diagnosed non-Hodgkin lymphoma cases in the US. Treatment advances include approval of oral agents such as ibrutinib and lenalidomide in relapsed/refractory MCL. In a study conducted among patients with chronic lymphocytic leukemia, treatment-related toxicity and associated discontinuation rates were higher in a real-world setting compared with clinical trials (Mato et al., Blood, 2016; 128:3222). However, similar real-world data are not available for MCL. Thus, this study aimed to explore and document current treatment patterns and associated adverse events (AEs) for MCL.
Methods: In thisretrospective cohort study, we used Truven MarketScan® databases, consisting of claims data relating to medical and drug utilization for more than 60 million unique individuals (in the most recent data year) across the US. Patients were included if they were age ≥18 years, had ≥2 medical claims on separate days with a diagnosis for MCL (ICD-9-CM code: 200.4x or ICD-10-CM code: C83.1x) between July 2012 and June 2015, and were enrolled in medical and drug plans continuously for ≥12 months before the study index date (baseline period), with no evidence of MCL diagnosis or prior MCL treatment in the baseline period. The date of first claim with MCL diagnosis defined the study index date. MCL-directed systemic therapy regimens, stem cell transplantation, and radiation therapy received after the study index date were identified using applicable procedure codes. Treatment regimens were defined as the combination of all treatments on and during the 35 days after the first claim for a systemic therapy agent. End-of-treatment episode was defined as the earliest gap of ≥90 days in treatment or a treatment modification (ie addition/subtraction of agents in the regimen or switch to a different regimen). Treatment patterns (eg duration of treatment, treatment sequencing, adherence to oral therapies) for up to 4 observed treatment episodes were analyzed. Occurrences of potentially treatment-related AEs were determined using AE-specific diagnosis and procedure codes and were stratified by the type of treatment. All analyses were descriptive in nature.
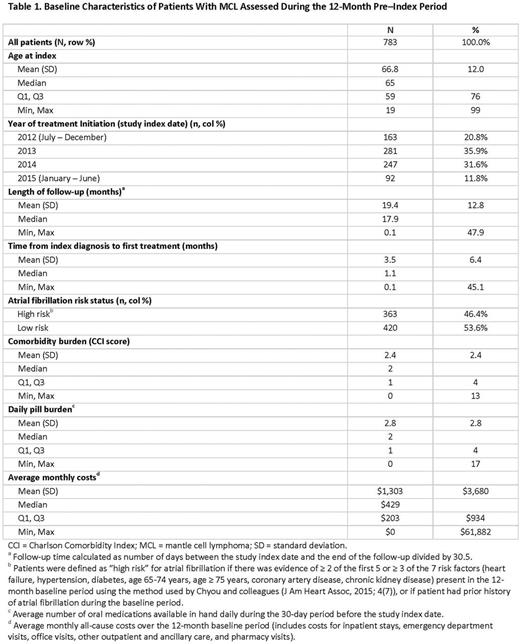
Results: A total of 783 patients with MCL met the study selection criteria; median age 65 years (range: 19-99). The median Charlson Comorbidity Index score assessed at baseline was 2.0 (range: 0-13) with a median of 2.0 (range: 0-17) daily oral medicines in the month before MCL diagnosis (Table 1). MCL-directed treatment was recorded for 636 patients (81%); among them, the treatments were chemotherapy (76%), biologic therapy/immunomodulators (64%), radiation therapy (32%), targeted therapy (17%), and stem cell transplantation (11%). Among those receiving systemic therapy at any time during the follow-up (all of above except patients undergoing radiation and stem cell transplantation; n=457), the most common regimens were bendamustine/rituximab (BR; 41%), rituximab/cyclophosphamide/doxorubicin/vincristine (R-CHOP; 27%), rituximab alone (including maintenance therapy; 20%), and ibrutinib alone (14%). Bortezomib- and lenalidomide-based regimens were used in 6% and 5% of patients, respectively. The median (Q1-Q3) duration of treatment in months for ibrutinib, BR, R-CHOP, and rituximab were 6.1 (3.4-9.9), 5.6 (3.2-9.3), 4.3 (3.0-4.9), and 2.4 (1.7-8.8), respectively. The most common hematologic AEs (includes both prevalent and incident cases) across the most common regimens were neutropenia and anemia. Infection, hypertension, dyspnea, and nausea/vomiting were common nonhematologic AEs. Among patients receiving ibrutinib, occurrences of neutropenia (17%) and pyrexia (6%) were less frequent, but atrial fibrillation (20%) and renal failure (14%) were more frequent, as compared with that in patients receiving the common chemoimmunotherapy regimens (BR and R-CHOP).
Conclusions: This study identifies the most commonly used MCL-directed treatments in a real-world setting. Chemoimmunotherapy combinations, particularly BR, remain the most common treatment choice for MCL. Ibrutinib is the most common novel agent used for MCL, with few patients receiving lenalidomide- or bortezomib-based therapies. The results indicate that the occurrence of AEs among patients with MCL receiving systemic therapy is substantial and that their frequency varies with treatment regimen.
Goyal: RTI Health Solutions: Research Funding. Karve: AstraZeneca: Employment, Other: Stocks. Nagar: RTI Health Solutions: Research Funding. Kaye: RTI Health Solutions: Research Funding. Mato: Portola: Research Funding; Janssen: Consultancy; AbbVie: Consultancy, Research Funding; Acerta: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy; AstraZeneca: Consultancy; Pharmacyclics: Research Funding; DTRM: Research Funding; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Regeneron: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.